Abstract
In response to the need to manufacture larger molecules for drugs than realistically can be made with conventional methods, biologics have quickly become more important in treating chronic illnesses. However, the oral bioavailability of biologics is generally only 1 – 2% due to harsh physiological challenges in the digestive track. One of the most challenging physiological barriers is the mucus membrane that covers the gastrointestinal (GI) tract which, by trapping biologic encapsulated particles, prohibits fast diffusion into cells and hastens their secretion from the body. The goal of this research is to determine which parameters inhibit mucus membrane penetration and design a delivery method to overcome this inhibition. An increase in bioavailability even to just 10% from 1-2% would vastly improve the economic viability of orally administering biologics, an obvious patient preference for comfort. To accomplish this goal, methods for tracking particles through the mucus membrane using pressure rather than diffusion will be developed. Fluorescent particles of varying size, density, and other variables will be delivered to ex vivo porcine intestines and analyzed in two major ways. With histological analysis, after delivering particles, tissue samples will be frozen, sectioned, stained, and imaged using both light and confocal microscopy techniques, allowing for the visualization of particles and determination of their location in the tissue. Through homogenization analysis, mucosal and cell tissue layers will be separated and homogenized to allow for quantitative measurements of particles and their locations after delivery. The use of these techniques will help determine how particle parameters (dose, size, density, etc.) and deliver method affect penetration of the particles through the mucus membrane and will aid design of better systems for the oral delivery of particle encapsulated biologics.
Unfortunately I cannot go into much more detail until research results are published. So here are some cool confocal microscope images I took. These are just controls, so there's nothing particularly interesting to point out.
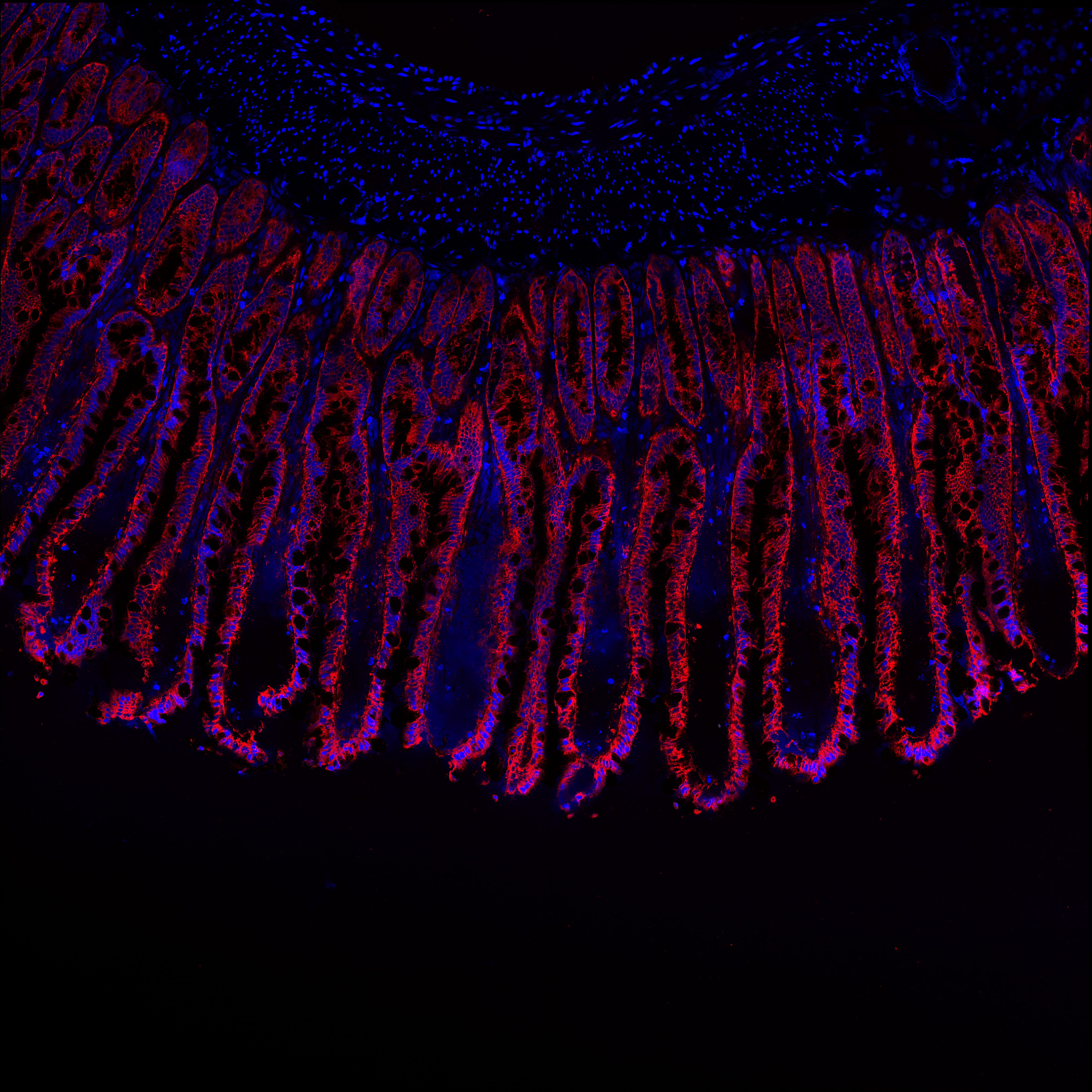
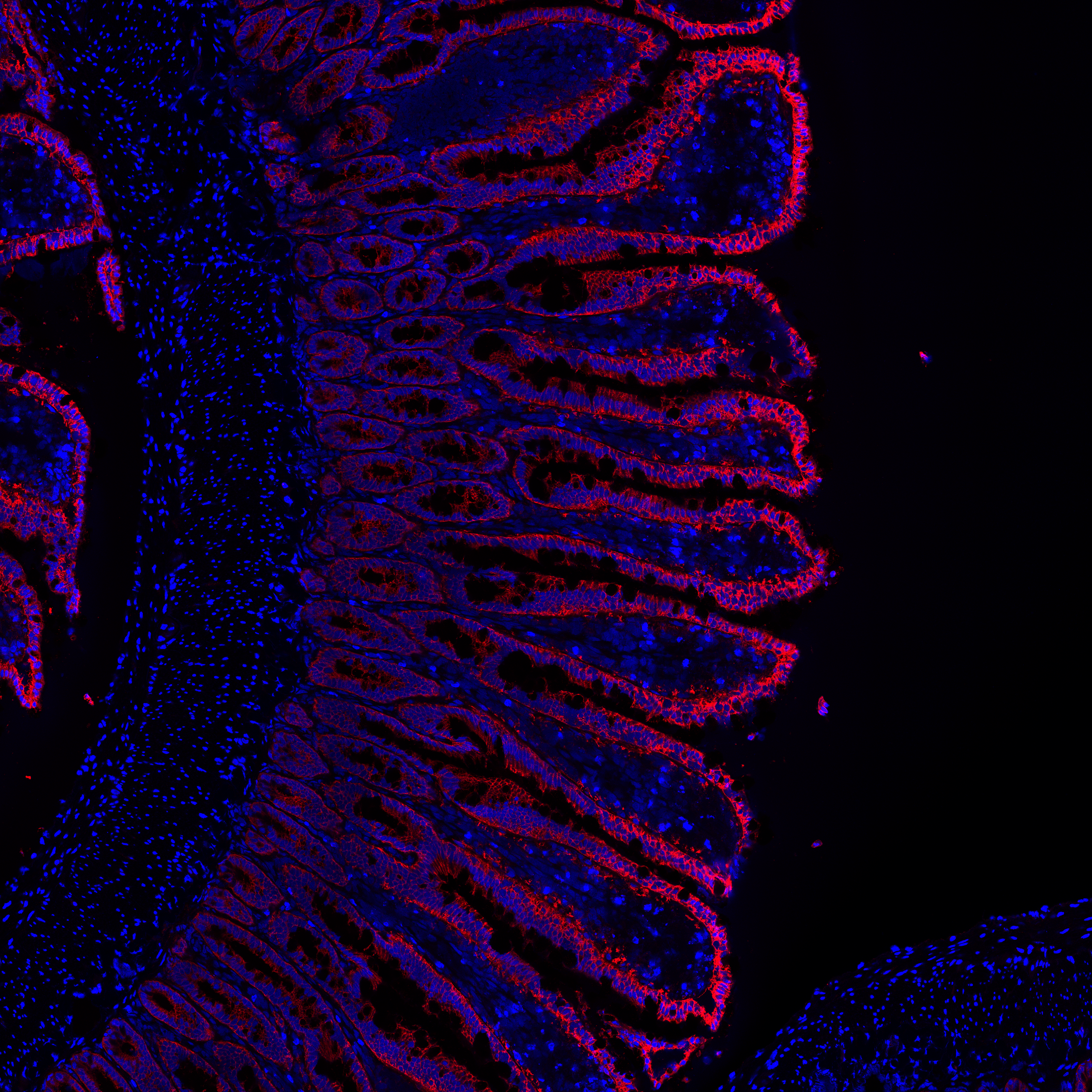
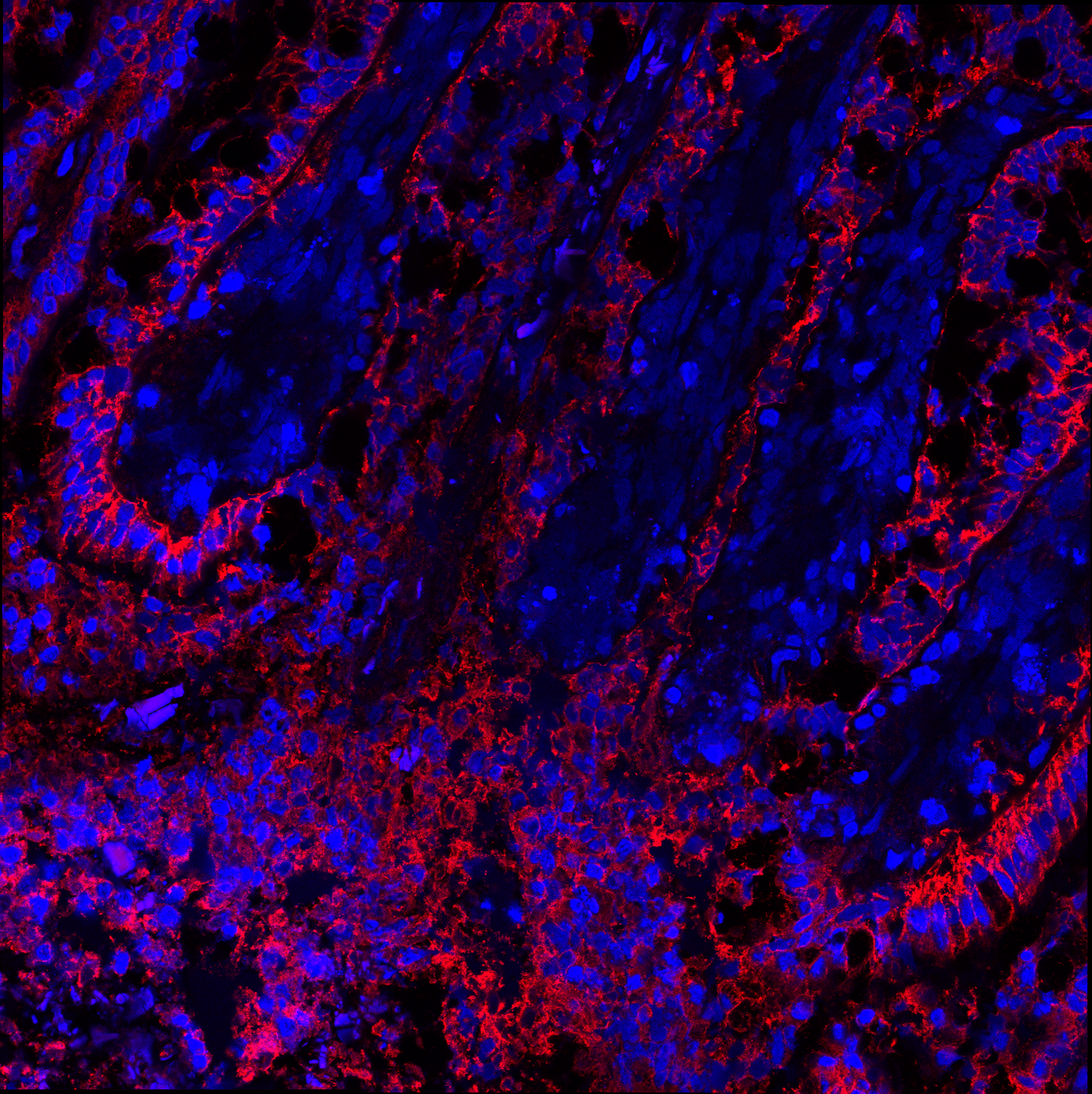
Images of villa from porcine jejunum. Control samples only.
Blue channel is DAPI, which stains cell nuclei. Red channel stains Muc2 (firmly adherent mucus of epithelium).
More Information
Here is a brief outline of what I did over my 3 years working in the lab.
- Created protocols for experimentation on porcine intestinal tissue.
- Iterated and improved design of active-force oral drug delivery method.
- Fully characterized operating parameters of novel drug delivery method and effects on mucosal layers and epithelium.
- Conducted histological analysis via frozen sectioning, fluorescent staining, and confocal microscopy.
- Wrote computer application to allow higher-level analysis of confocal images.
- Fully characterized physical parameters of device material function in acidic GI environment.
- Programmed scripts in MATLAB to automatically analyze and organize experimental data.
- Developed mathematical model from fundamental theory describing material characterization - focusing primarily on fluid mechanics and diffusion.
- Gained approval for live animal testing on rats, and then conducted this study through surgeries.